Training TWO Guide
Section ONE: (Est. Time = 15 min)
What is the signal?
There is a “Director’s Briefing icon” linked to video instructions for the section so the students know exactly how to do the work.
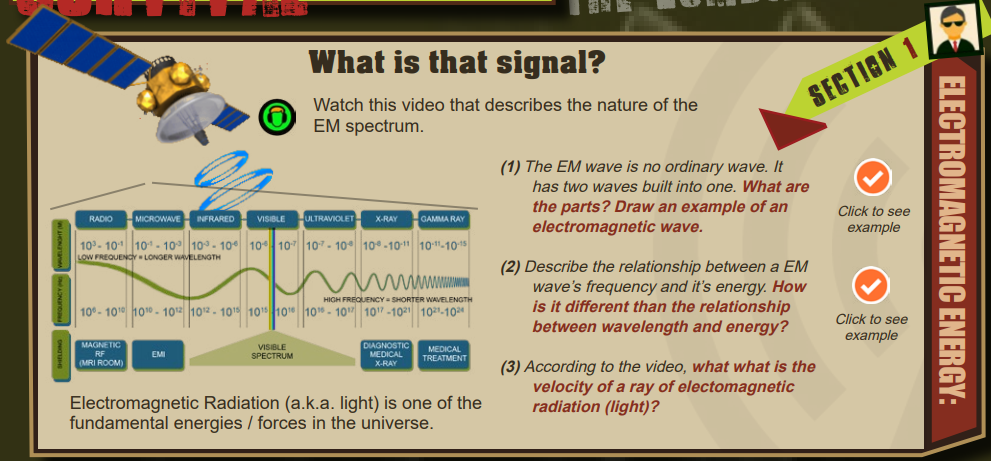
The mathematical applications of quantifying EM waves begins in this training. The inverse relationships between wavelength and frequency (and energy) are emphasized numerically as well as conceptually. “Check it out” icons are presented here to provide students with convenient visual examples of EM wave structures and visual examples of how shorter wavelengths mean higher frequencies (and higher energy).
The media icon is set to present a video by “Professor Dave” an internet science explainer about EM waves and light energy. (This would be a good place to start if teaching in person) For more videos by “Professor Dave Explains”, click here.
Answers to Section One Questions:
- The two parts of an EM wave are the electrical wave component and the magnetic wave component. (The drawing should be similar to the image provided by the “check it out” icon to the right.
— The relationship between frequency and energy is direct. If one goes up, the other also goes up. The relationship between wavelength and energy is inverse. If one goes up the other goes down in numeric value.
—The velocity of a ray of light is 186,000 miles/sec or 300,000,000 meters/sec or 300,000 Kilometers/ses15
Section TWO: (Est. Time = 35 min)
Every wave is made of two parts:
There is a “Director’s Briefing icon” linked to video instructions for the section so the students know exactly how to do the work.
The ability to DO MATH is a critical part of science learning. The equations to be used here are presented in the FORMULAS box. The box is designed to be a quick way to find the math formula for every variable that would need to be solved. For example, if frequency needed to be solved for the equation would = C/wavelength (in meters).
A calculator icon is linked to an online scientific calculator for students to use in another open tab. (Access by right-clicking the icon and choose, Open in a new tab. Then both the PDF and the calculator are open in the same window.)
The media icon at the bottom links to a 8:57 min. long video that provides walk-through examples of how to do the math problems.
For additional practice, or extra credits or achievement – the “Check it out” icon links to a document with more practice problems to try.
Answers to Section One Questions:
- 24 mm -> 24×10-3 m -> Freq = 3 x108 / 24×10-3 m =
3.00 x108 Hz - 1.2 micrometers -> 1.2×10-6m -> Freq = 3 x108 / 1.2×10-6 m =
1.86 x1014 Hz - 5.3 x10-7 m -> (no conversion needed) Freq = 3 x108 / 5.3×10-7m =
5.66 x1014 Hz - 8.5 x10-8 m -> (no conversion needed) Freq = 3 x108 / 8.5 x10-8 m =
3.53 x1015 Hz - 654 nanometers -> 654 x10-9 m Freq = 3 x108 / 654 x10-9 =
4.59 x1014 Hz
- 23,000 Hz -> (no cnv needed) -> WL = 3 x108 / 2.3×104 m =
13,000 m - 3.4 MHz -> 3.4×106Hz -> WL = 3 x108 / 1.2×10-6 m =
88.2 m - 5.1 GHz -> 5.1×109Hz WL = 3 x108 / 5.1×109Hz =
0.059 m or 5.9 x10-2 m - 7.75 x1016 Hz -> (no cnv needed) WL = 3 x108 / 7.75 x1016 Hz
3.87 x10-9 m - 5.31 x1019 Hz -> (no cnv needed) Freq = 3 x108 / 5.31 x1019 Hz
5.65 x10-12 m
(If given wavelength, frequency should be calculated first)
- Freq = 4.5x1015 Hz -> E = 6.636 x10-34 x 4.5x1015 Hz =
2.99 x10-18 Joules (J) - Freq = 9.3x105 m -> = 6.636 x10-34 x 9.3 x105 Hz =
6.17 x10-28 Joules (J) - WL = 2.4 x10-11 m -> Freq = 3 x108 / 2.43x10-11 Freq = 3.23 x1012 Hz
E = 6.636 x10-34 x 3.23x1012 Hz =
8.28 x10-15 Joules (J) - WL = 6.1 x10-16 m -> Freq = 3 x108 / 6.1 x10-16 m = 4.9 x1023 Hz
E = 6.636 x10-34 x 4.9 x1023 Hz =
3.3 x10-10 Joules (J)
Section THREE: (Est. Time = 25 min)
The source of all light – it’s all made the same.
There is a “Director’s Briefing icon” linked to video instructions for the section so the students know exactly how to do the work.
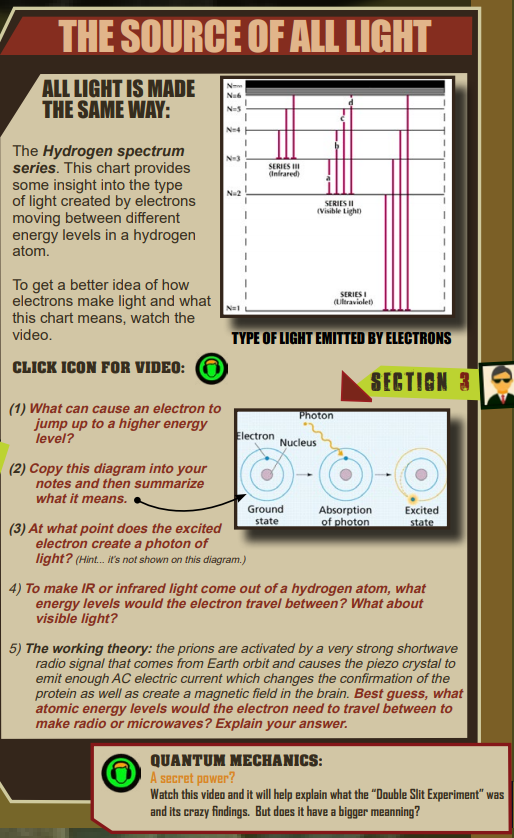
One of the more foundational parts of chemistry is the introduction into the quantum mechanical nature of electrons. The movement of electrons is described as being “instantaneous” where excited electrons will move from one energy level to another via a process referred to as quantum tunneling. The first figure at the top of the section is the hydrogen spectrum which shows the range of electromagnetic radiation that is emitted from an atom when an electron starts as a certain level, rises to an excited state, and then returns to that level.
The media icon is linked to an extensive 14:14 min long video on the excitation process for students that would like to have a more in depth presentation of the information.
As a bonus, there is a media icon at the bottom of the section that talks about the connections between quantum mechanics, the double slit experiment, and possibly the hint our undiscovered human superpowers?
Answers to Section One Questions:
- Any form of energy, like another photon of light or environmental heat (kinetic) energy that has the right amount of energy to move the electron from its resting ground state to an excited state.
- (Student copies the drawing into their notes as presented.)
- The tough part about this diagram is that the part that creates light is when the electron returns back to it’s ground state position. Only if told or if the student watched the video would they know that.
- For an atom to release or emit IR type EM waves, the ground state of the electron would have to be N = 3 (as seen from the diagram at the top.)
- This is a theory and open ended. Credit should be given if the student realizes that the piezo crystal reacts to radio waves or possibly low energy microwaves. If they state the electron ground state should be either N=5 or N=6, that would be awesome.
Section FOUR (Est. Time = 35 min)
The source of all light – it’s all made the same.
There is a “Director’s Briefing icon” linked to video instructions for the section so the students know exactly how to do the work.
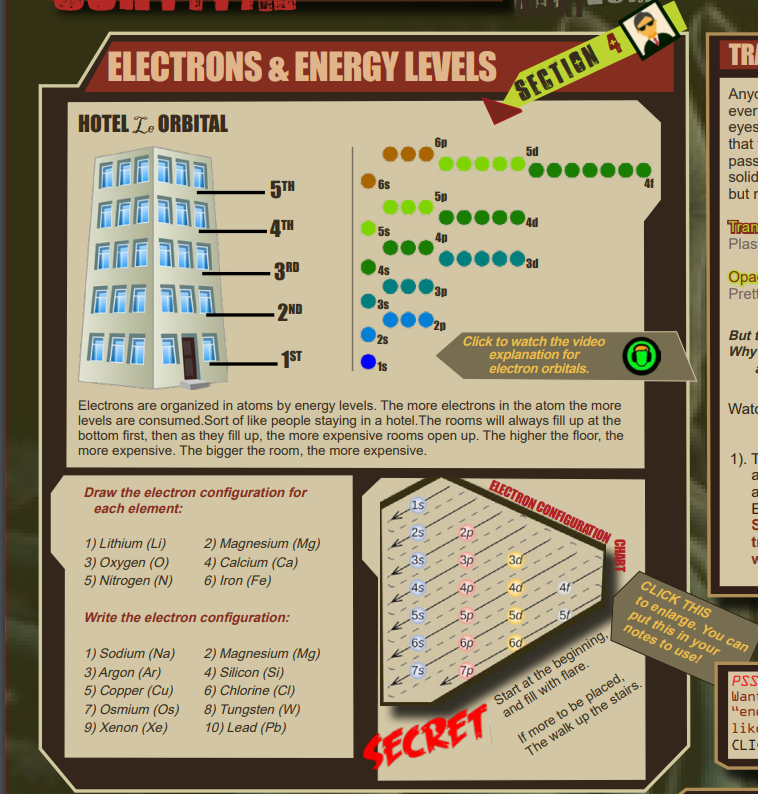
The term location is used almost metaphorically because the exact location of any electron around an atom is almost impossible to acquire. As such, most of the data about the electrons’ “location” is done so in terms of its energy level which are based on the quantum numbers.
The diagram at the top is designed to make a metaphor in the students heads that a possible way to organize or conceptualize atomic energy levels is to think of a hotel, albeit, a very weird hotel. The media icon here is linked to the whole sorted story on how to “find the homes” of each of the electrons in an atom.
Answers to Section One Questions:
[DRAW THE ELECTRON CONFIGURATIONS.]
Click here to get the hand-drawn key for Section 4 part 1
[WRITE THE ELECTRON CONFIGURATIONS.]
Click here to get the hand-drawn key for Section 4 part 2
Section FIVE (Est. Time = 15 min)
The source of all light – it’s all made the same.
There is a “Director’s Briefing icon” linked to video instructions for the section so the students know exactly how to do the work.
Section 5 revisits the idea of energy levels and how “FALLING” electrons are the driving force behind photon emission.
The answers here are all about things falling and the idea that the higher something falls from the more energy it has. Which, in a sense, is what we are suggesting electrons do in a metaphorical sort of way.
Answers to Section One Questions:
- If the zombie falls out the top floor to the ground he will hit with a lot of force energy, just like how an electron falling from N=5 would produce an electron of high energy.
- This could be a tricky question and the real important part about this is that not all the floors are the same distance from each other. ANYTHING falling to the first floor will have a longer way to go (have more energy) than any thing falling from any other floor to any other floor.
- According to the EM chart at the top of Training 2, the highest energy photo in the list is visible light. Therefore, the the electron must be falling from the highest distance when making visible light.
Bonus section (Est. Time = 20 min)
What makes something transparent?
One of the great questions of our time, what makes something invisible or see-through?
The media icon is linked to a video that demonstrates how electrons are entirely responsible for this phenomenon.
The second media icon is a link to a “sawwweeeeet” video on the mathematical representations of electron orbitals… as they would actually appear! It’s worth a looksy.
Answers to Section One Questions:
- The crux of this idea centers around the concept of quantized energy. There is an exact amount of energy that an electron needs… no more… no less.. to move between energy levels. If it gets that energy, the photon of visible light will be absorbed and the light CANNOT pass through. If the energy of the photon is too low to be absorbed, the light CAN pass through any solid object as if it wasn’t even there. That’s awesome.