Section ONE: (Est. Time =15 min)
The thinly drawn shade & and the foreshadow of disaster.
NOTE: There are no Director Briefing Icons on these documents at this time. More explicit directions will need to be given to the students.
Outside the context of San Jacinto, this doesn’t make a lot of sense. I would look at this as a sort of example. A trial that uses this area as a practice for securing a parameter. Once these have been used as examples, then students could engage in finding their own neighborhoods on Google Maps to identify areas where breeches could happen.
Links to coordinates on Google Earth have been linked to each red area, they all bring up different parts of this city.
But… why? Once the breech points have been identified they can be sealed in such a way that encourages an enemy to take a specific path that takes them to our field of creatively applied alkali metals.
Answers to Section 1 Questions:
- Ask the students to identify areas that they feel would be “secure”. Ask them to explain why. Reasons can vary as they likely do not have much in the way of expertise in this area.
Section TWO: (Est. Time =25 min)
Bunker Street: Alkali Metals Put to Work.
NOTE: There are no Director Briefing Icons on these documents at this time. More explicit directions will need to be given to the students.
Outside the context of San Jacinto, this doesn’t make a lot of sense. I would look at this as a sort of example. A trial that uses this area as a practice for securing a parameter. Once these have been used as examples, then students could engage in finding their own neighborhoods on Google Maps to identify areas where breeches could happen.
Figure Chi (The X thingy): Shows that Alkali Metals have the largest atomic radius of all of the elements on the periodic table.
The attractive force of the last electron gets less and less as the atoms get larger in diameter. FOR THAT REASON, larger alkali metals are more reactive than smaller ones.
Answers to Section 2 Questions:
- Draw the chart in the ATN – should be min 1/3 of a page.
— - The connection is The larger the atom, the more vigorous the reaction.
— - The last electron, also known as the “valence electrons”
— - Oxidation is a chemical process that adds the atom oxygen to the metal to form a compound called an “oxide”. Adding oxygen to a metal atom is also more commonly known as rusting.
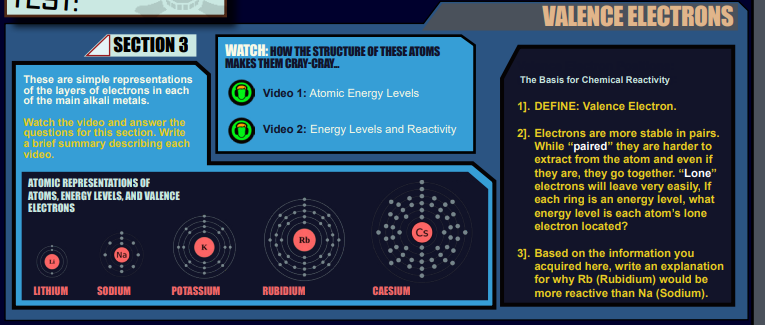
Section THREE: (Est. Time =15 min)
Valence electrons – the only electrons that ever matter… for anything, ever.
NOTE: There are no Director Briefing Icons on these documents at this time. More explicit directions will need to be given to the students.
The videos provided here are key to the understanding of valence electrons. Watching as a group might be the more ideal way of addressing the content. Stop and address key elements of valence electrons – Lone electrons are more likely to leave, farther from the nucleus are more likely to leave, paired electrons are more likely to stay put.
OPT: Students draw the Bohr model atoms in their ATN. Focusing on and perhaps labeling the last electron as the valence electron.
Answers to Section 3 Questions:
- Define: Valence electron(s) are the electrons that occupy the highest energy orbital and represent the population of electrons that would most likely participate in chemical bonding or ionization.
— - For alkali metals, the lone electron is located in the last energy level, the valence shell.
— - Rb (rubidium) is more reactive than Na (sodium) because the valence electrons are located farther away from the nucleus. They experience less attractive force from the nucleus and as such require far less energy to leave to form bonds with other atoms.