Training ONE Guide
Section ONE: (Est. Time = 40 min)
The terror agenda goes nuclear
There is a “Director’s Briefing icon” linked to video instructions for the section so the students know exactly how to do the work.
The goal of this section has the student focusing on using the Reduction table to figure out the cell potentials for the materials listed. Find the (3) three highest anode cathode combinations for the metals given here.
The “Media Icon” plays a video that describes how to use the reduction table. Plus if students click on the reduction table a larger version appears for them to use.
Answers to Section 1 Questions:
- #3 Best ECell value = Anode (Mg) & Cathode (Cu) ECell = 2.7 volts
— - #2 Best ECell value = Anode (Al) & Cathode (Cu) ECell = 1.8 volts
— - #1 Best ECell value = Anode (Mg) & Cathode (Cu) ECell = 0.5 volts
Section TWO: (Est. Time = 10 min)
More Power! Electro-chemical Cells
There is a “Director’s Briefing icon” linked to video instructions for the section so the students know exactly how to do the work.
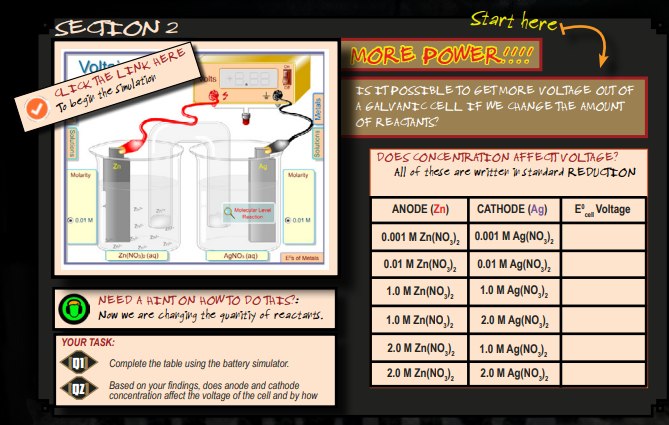
The goal of this section is to have the students participate in the virtual lab. The combinations of Anodes and Cathodes are provided on the PDF. The students provide the ECell voltage for each combination.
The “Media Icon” icon will provide to the students instructions on how to access and use the simulation.
Answers to Section 1 (first part)Questions:
- Student draws the table into their ATN and then progresses to fill it out. All of them should be the same. A sample of a table filled out can be seen here. Click here.
— - The concentration has an effect on the concentration, the higher the concentration of chemicals that
Section THREE: (Est. Time =40 min)
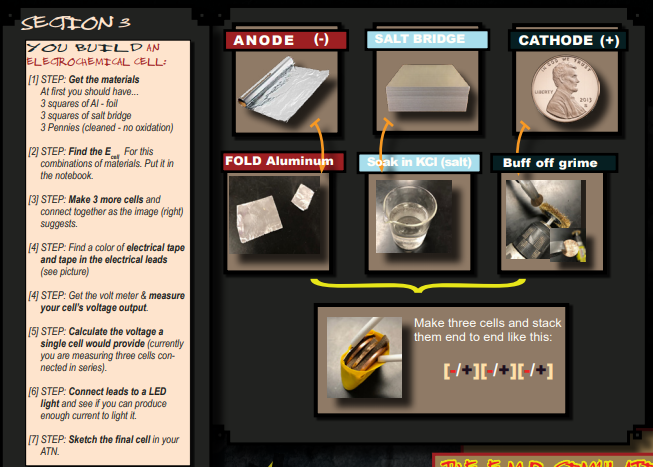
The Build: Making an electro-chemical cell.
There is a “Director’s Briefing icon” linked to video instructions for the section so the students know exactly how to do the work.
This section is specifically designed to be an adventure of discovery. Students will use the reductions potential chart and their previous training
Answers to Section 3 Questions:
- Fission is the breakdown of large particles that release energy. Fusion is the combination of smaller elements to make larger ones. The loss of neutrons in the fusion process is what is converted to heat energy.3
Section FOUR: (Est. Time = 25 min)
Could You Charge It?
There is a “Director’s Briefing icon” linked to video instructions for the section so the students know exactly how to do the work.
The “Media icon” presents the students with a video that describes some of the basics of nuclear chemistry. The video is a YouTube video presented by Crash Course and is part of their chemistry series. More Crash Course vids can be see by visiting their channel by clicking here.
As a side note there is a secret buried in this section. Click around and see if you can find it.
Answers to Section 4 Questions:
- Radioactive decay occurs when either chucks of nucleus, electrons or electromagnetic radiation are emitted from an atomic nucleus in a spontaneous manner.