Training TWO Guide
Section ONE: (Est. Time =20 min)
Massive atoms make massive gravity.
There is a “Director’s Briefing icon” linked to video instructions for the section so the students know exactly how to do the work. There’s some explaining to do with this first section, so please watch it as a group or encourage them to watch it on their own.
To understand how this massive elements could be possible, one has to get a solid feel for how the little ones are constructed. FIRST, the students will copy this table into their ATNs – should take up about half a page.
Students will watch the media icon video called the “island of stability”. It’s a video from a while ago that predicted an area of the periodic table where super massive elements could be stable and allow for the creation of incredible materials.
To provide the data for the table, three check-it-out links are provided that take the student to pages where data can be acquired about each of the elements… except element 115. That one has to be predicted by assessing the ratios of protons, neutrons and masses, of the other elements to project estimations for Moscovium.
Answers to Section 1 Questions:
Fill out the grid. To see what a correct version of the grid looks like filled out, Click here.
Questions:
- The trend can be seen by noticing the ration of neutrons to protons increases as the the elements get larger. The larger the atom, the more neutrons it takes to keep the nucleus from flying apart.
— Neutrons are essentially the glue or the adhesive that makes the protons able to stay locked into the nucleus.
Section TWO: (Est. Time = 30 min)
Various ways to find density
There is a “Director’s Briefing icon” linked to video instructions for the section so the students know exactly how to do the work. There’s some explaining to do with this first section, so please watch it as a group or encourage them to watch it on their own.
Students will watch the media icon videos that each demonstrate a different method for discovering (calculating) the density of an object. These are diagnostic tools that come up later in the “Examulation” and are important to know.
The goal here is to practice calculating the density of an object that incorporates three different methods.
Answers to Section 2 Questions:
Samples of worked out problems: Click here
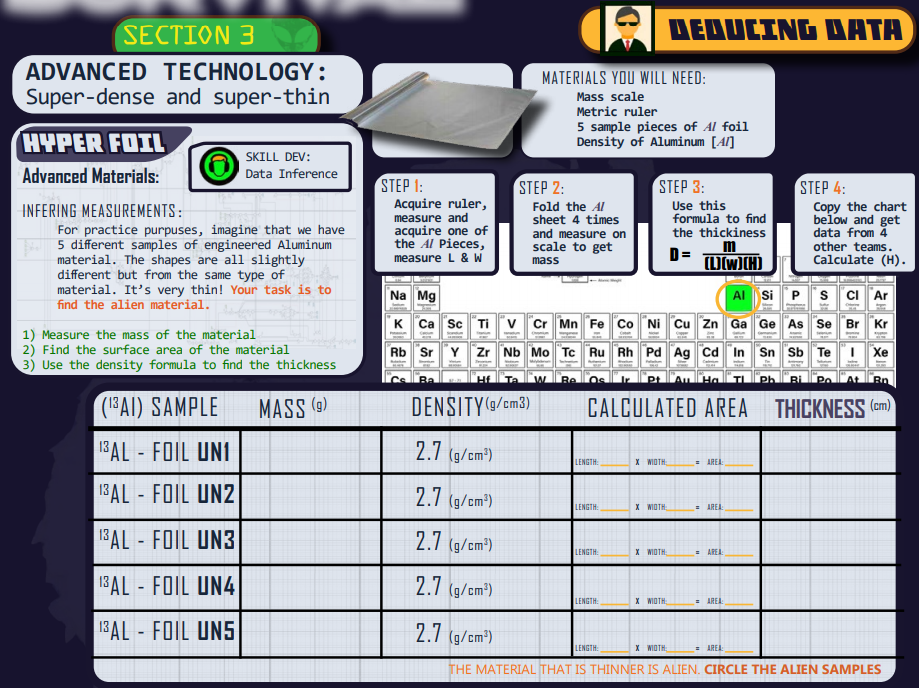
Section THREE: (Est. Time = 35 min)
Using data for deduction
This is a hands-on learning experience. As such, clicking on and listing to the “Director’s Briefing icon” is going to contain much needed information about the goals for the students.
The media icon video has a “how to” video describing how data can provide agents or whomever the ability to infer conclusions.
The table is filled out by the students and put in their ATN.
The GOAL: Calculate the thickness of the aluminum foil when there is no measurement tool that can actually measure it.
Answers to Section 3 Questions:
To see a sample of what the grid might look like and example problems: Click here
Section FOUR: (Est. Time = 20 min)
High Tech Materials
This section is more traditional research gathering. If this “false flag” operation were to appear real, then the materials they use are going to be unlike anything we’ve ever seen. “Director’s Briefing icon” is going to guide the agents on what to do in this section.
The media icon video has a video that describes the only person alive to have “allegedly” worked with element 115 in it’s stable state. That guy is talked about in this video.
This section also broaches the topic of chemical bonds. The “Check it out” icon is linked to additional sources on that topic. Access other icons like this in part three that present brand new discoveries in ultra-light compounds.
The GOAL: Collect the needed information in each group of materials. Put the answers in full sentences in the ATN of each agent.
Answers to Section 4 Questions:
Ultra HARD:
- The atoms in the diamond are arranged in a tetrahedral geometric shape. This contributes to the diamonds hardness.
—- - The tetrahedral structure appears to have a more tightly packed group of atoms.
Ultra DENSE:
- Other elements in the same column as Mc would be Bismuth, Nitrogen and Arsenic.
—- - The only evidence that suggests that it could exist in a stable form is from Mr. Lazar’s testimony. According to him, this sample was not from this solar system and could not have been made in this solar system at the very least, not made on Earth.
Ultra LIGHT:
- The atoms of the ultra strong atoms are very highly organized whereas the atoms of the very light materials are amorphous,
disorganized, chaotic, there’s no real structure in their appearance.
—- - The material almost floats in air because the substance is mostly empty space. Air fills the majority of the structure.