Training ONE Guide
Section ONE: (Est. Time =30 min)
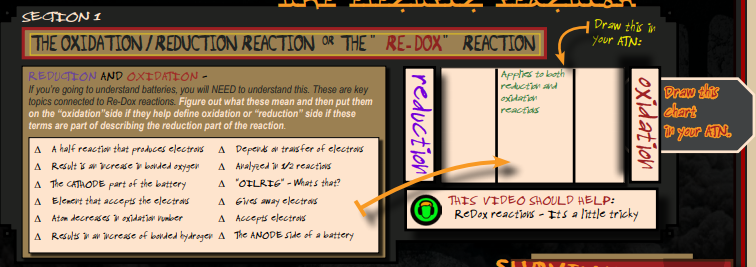
Oxidation vs. Reduction
There is a “Director’s Briefing icon” demonstrates how to do this organizational concept map.
The section is done in two sections, (1) Watch the video to become familiar with the concepts, (2) put them in the appropriate part of the diagram as either related to the reduction side of the battery or the oxidation side, or does the term have influences from both sides?
The “Media icon” takes the students to a “Crash Course” video on YouTube. The video describes what a Re-Dox reaction is and explains what happens on each side of the battery.
More “Crash Course” videos can be acquired and viewed through their channel on YouTube, click here to view.
Answers to Section 1 Questions:
- The half-reaction that produces electrons – this is the definition of oxidation and would go on the oxidation side only.
— - Result in an increase in bonded oxygen – Oxidation side (increase in bonded oxygen is where the “oxidation” term was derived. Think of rust.
— - Cathode is the reduction side, it accepts the incoming electrons.
— - The element that accepts the electrons would be on the reduction side.
— - Atom that decreases in oxidation number would be the reduction side “reduces” leads to the term reduction side.
— - Results in an increase in bonded hydrogen – Reduction side.
— - Depends on transfer of electrons – Both sides (place in middle)
— - Analyzed in 1/2 reactions – Both sides are (place in middle)
— - OILRIG – goes in the middle as it talks about what happens to both sides oxidation side and reduction side.
— - Gives away electrons – Oxidation side
— - Accepts electrons – Reduction side
— - The ANODE is – Reduction side
Section TWO: (Est. Time = 25 min)
The Electrochemical cell
There is a “Director’s Briefing icon” is not very long as this section is meant only to have the students reproduce a diagram of the simple galvanic cell in their ATN.
The goal of this section is to have the students (agents-in-training) re-create the image here in their ATNs. The sections should be labeled and the image be at least half a page in size. If the areas of the cell are not clearly labeled the student should be encouraged to fix or adjust the image so that the information is more clear.
The “Media icon” takes a deeper look at batteries using the “Crash Course” videos resource again. The video presents the concepts of voltage as well as the visualization of how batteries function.
More “Crash Course” videos can be acquired and viewed through their channel on YouTube, click here to view.
Answers to Section 2 Questions:
- The chart should have covered 1/2 a page and been labelled with all of the items shown in this section.
Section THREE: (Est. Time =20 min)
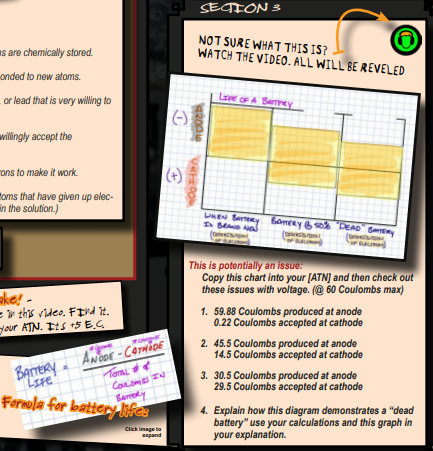
Nuclear vs. Thermonuclear
There is a “Director’s Briefing icon” If possible, this director’s icon should be watched as a group with pauses and talking about what this chart means.
The “Media icon” provides more information on what this chart means. The confusing part about this diagram is the fact that there appears to be electrons left over in the “dead batter”. The fact here is that the battery runs on a DIFFERENCE of electrical charge – the voltage is the difference in electrical charge. When the voltage = 0 the batter is dead and it quantity of electrons looks like it would in the chart here.
There is also a graphic to the lower left that has the formula used to calculate battery life. (I just noticed this, and there is a mistake in the video towards the end – maybe share some EC if someone finds the mistake?)
Answers to Section 3 Questions:
- First, the students copy the image down in to their notebook. This provides more “cognitive time” to grapple with the concept.
— - If the total number of electrons in the anode when the batter is new is 60 Coulombs, then take (59.88-0.22) / 59.88 = 99%
— - Here’s the math, (45.5-14.5) / 48.5 = 63%
— - Here’s the math, (30.5-29.5) / 30.5 = 1.6%
— - In the last section the difference between the anode and the cathode is 0. 0 / 30 is zero. There is no difference and zero percent difference remaining in the battery.
Section FOUR: (Est. Time = 25 min)
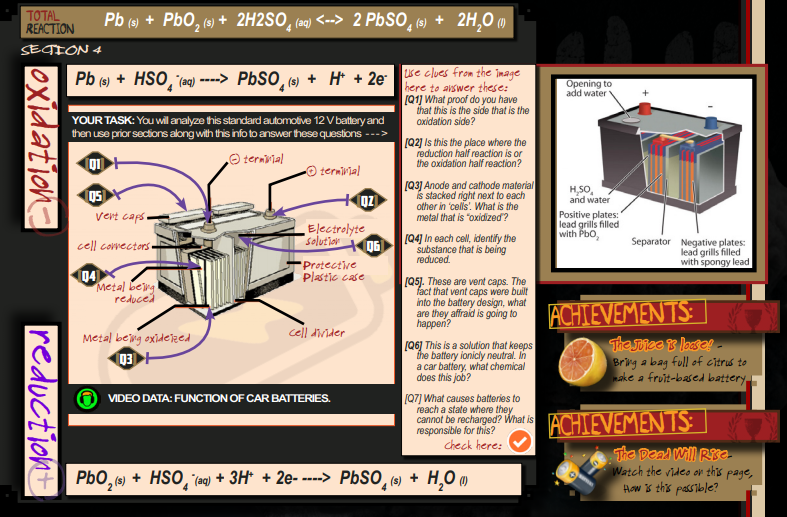
The Lead-Acid Car Battery – Applied ReDox
There is a “Director’s Briefing icon” Seek it out so that you can know what to do here! Though it’s not complicated. See the parts, try to figure out what they do.
The “Media icon” plays a video that demonstrates how car batteries function. It would be a great idea for this to be viewed first. It’s on YouTube – no once fancy, just a solid video that does a good job of describing how these batteries function.
The OXIDATION SIDE (the anode) half reaction is at the top of the image. The REDUCTION SIDE (the cathode) half reaction is at the bottom.
On the upper right side of the section is a graphic that demonstrates the internal workings of the lead-acid battery
Leader Board: There are some leader board achievements here that could really benefit the students in the long run! There is some likelihood that the students already have some experiences with it as well.
An additional link to “Battery University” for more background on batteries. Click here.
Answers to Section 4 Questions:
- Proof = The oxidation end is the (-) terminal as indicate in the image.
— - On this side, the (+) terminal is where the reduction half reaction occurs.
— - (using the half reaction for oxidation) the metal that is oxidized is Pb (lead).
— - (-) Oxidation side, the part that is being reduced is the HSO4– ion.
(+) Reduction side, the part that is being reduced is the PbO2
— - The vent caps are there to ensure the water pressure inside the battery doesn’t cause the battery to rupture and spray sulfuric acid all over the engine or some person.
— - The electrolytic solutions (similar to a salt bridge) keeps the solution electrically neutral.
— - (The answer to this questions requires the use of the “Check it out” icon next to the question.) The battery reaction stops “recharging” or running backwards, when the Sulfate ions start to crystallize on the negative plate. As they do this, less usable reactant floating around in the solution to use. As a result, the car battery no longer holds a charge. Because the reaction will not go backwards sufficiently enough to create the number of electrons (Coulombs) to start the engine.
Section FIVE: (Est. Time = 40 min)
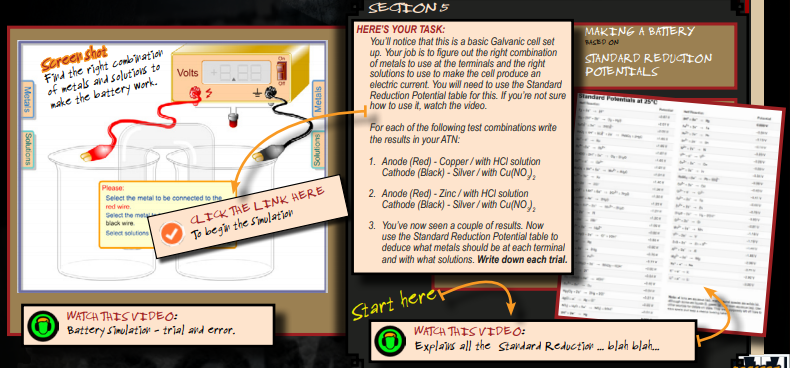
Wanna Play Battery?
There is a “Director’s Briefing icon” basically guides you to the green “Media Icon” which is a video that shows you how to work the simulation.
This section is sort of like a mini-lab. The task for this section is to vary the anode, cathodes, and electrolytes to achieve the highest charge possible. Follow the steps.
The biggest helper to this is the Standard Reduction Potential table. There is a “Media Icon” available that leads to a video which sheds some light on how to use the table to figure out the simulation.
Answers to Section 5 Questions:
- Student follows the directions and then writes down the results for each trial.