Training THREE Guide
Section ONE: (Est. Time = 30+ min)
Massive atoms make massive gravity.
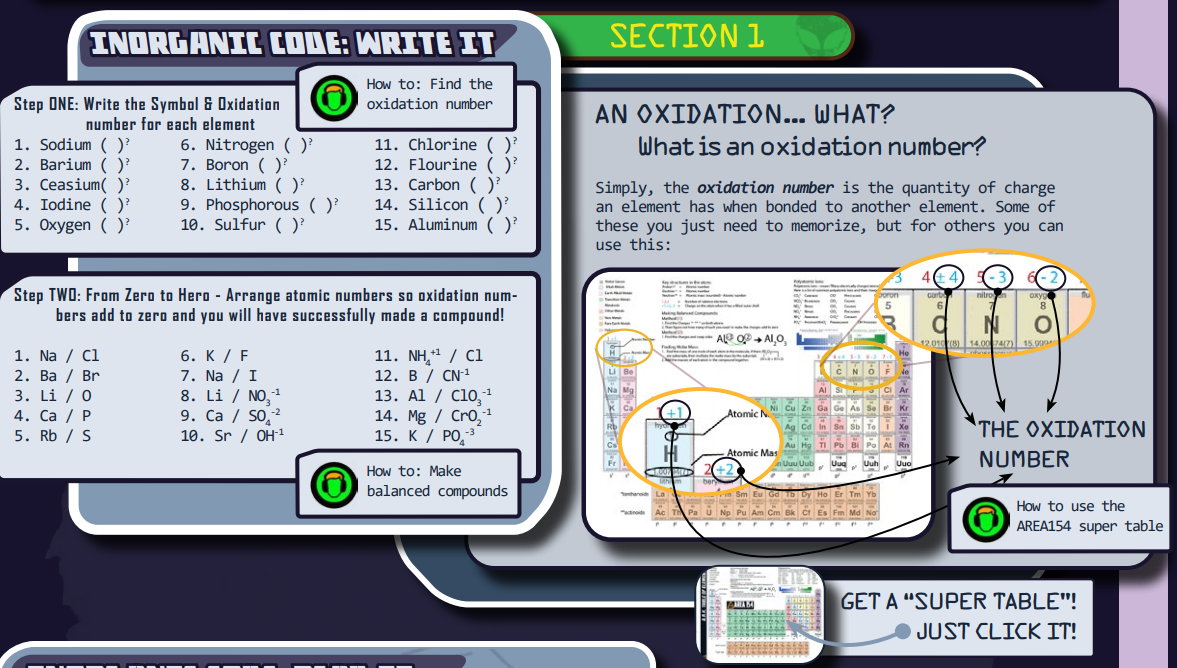
There is a “Director’s Briefing icon” linked to video instructions (not pictured here, but it’s on the PDF). It will provide the instructions on how to do Section 1. There is a lot here… and those who have taught chemistry will know.
The primary focus for this section is to grasp and demonstrate the concept of an oxidation number (sometimes referred to as the “charge”). In the first part the student writes the atomic symbol & and the oxidation number.
The green Media Icons will demonstrate how to accomplish finding the oxidation numbers for each element by doing several examples. This is true for both parts. The Media icon by the periodic table will provide instructions on how to read the AREA154 periodic table. To get your own copy of the table, just click the pic below the gray box.
Answers to Section 1 Questions:
Students will fill out both of these sections in their ATN. To see a completed version of both parts of section one:
- Click here (Part 1)
- Click here (Part 2)
Section TWO & THREE:
(Est. Time = 30 min)
Massive atoms make massive gravity.
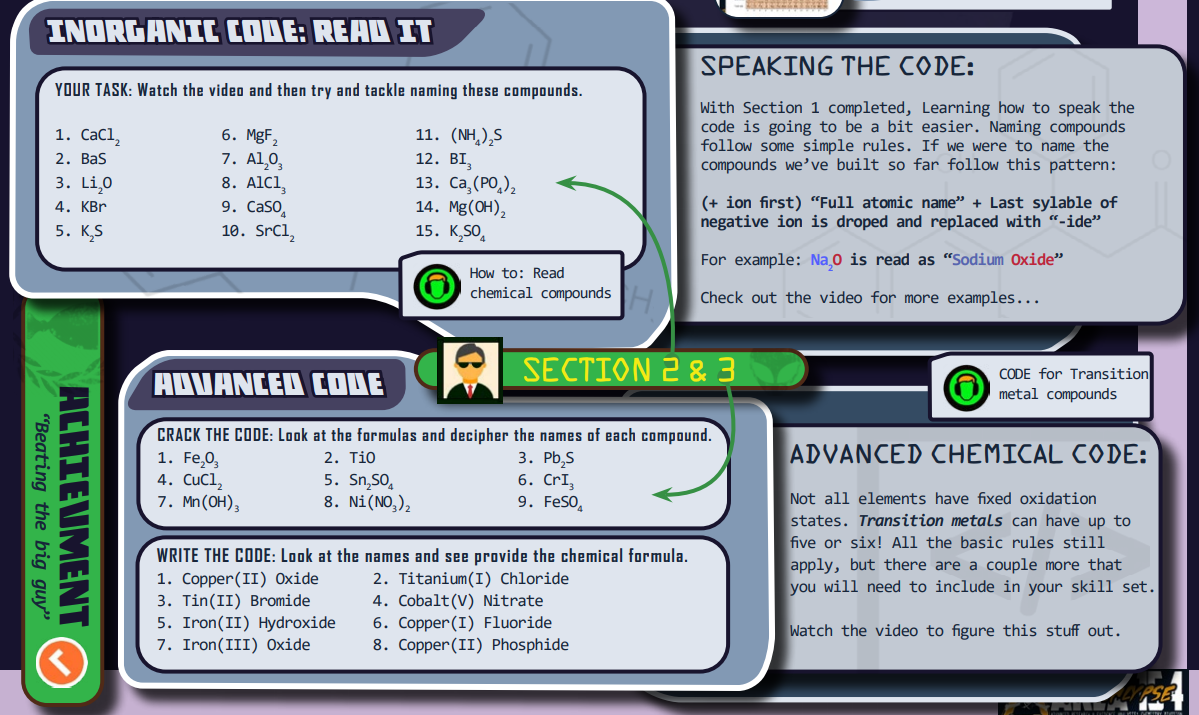
There is a “Director’s Briefing icon” linked to video instructions for the section so the students know exactly how to do the work. There’s some explaining to do with this first section, so please watch it as a group or encourage them to watch it on their own.
Answers to Section 2 Questions:
Students will fill out both of these sections in their ATN.
To see a completed version of both parts of section one:
Calcium Chloride
Barium Sulfide
Lithium Oxide
Potassium Bromide
Potassium Sulfide
Magnesium Fluoride
Aluminum Oxide
Aluminum Chloride
Calcium Sulfate [The -ate ending says this is a “group” ion]
Strontium Chloride
Ammonium Sulfide
Boron Iodide
Calcium Phosphate [The -ate ending says this is a “group” ion]
Magnesium Hydroxide
Potassium Sulfate [The -ate ending says this is a “group” ion]
Answers to Section 3 Questions:
Students will fill out both of these sections in their ATN.
To see a completed version of both parts of section one:
(Part I) Click here
(Part II)
Iron (III) Oxide
Titanium (I) Oxide
Lead (I) Sulfide
Copper (II) Chloride
Tin (II) Sulfate [The -ate ending says this is a “group” ion]
Chromium (III) Iodide
Manganese (III) Hydroxide [Hydroxide is a group ion]
Nickel (II) Nitrate [The -ate ending says this is a “group” ion]
Iron (II) Sulfate [The -ate ending says this is a “group” ion]