[AI:22] Balanced Chemical Formulas Part 3
We started on Monday with learning about oxidation numbers and how they work together to form balanced chemical compounds. Today we finish that part and open the door to the next step… taking the formula and then naming them!
TODAY IN AREA154:
Open the website in your Chromebook:
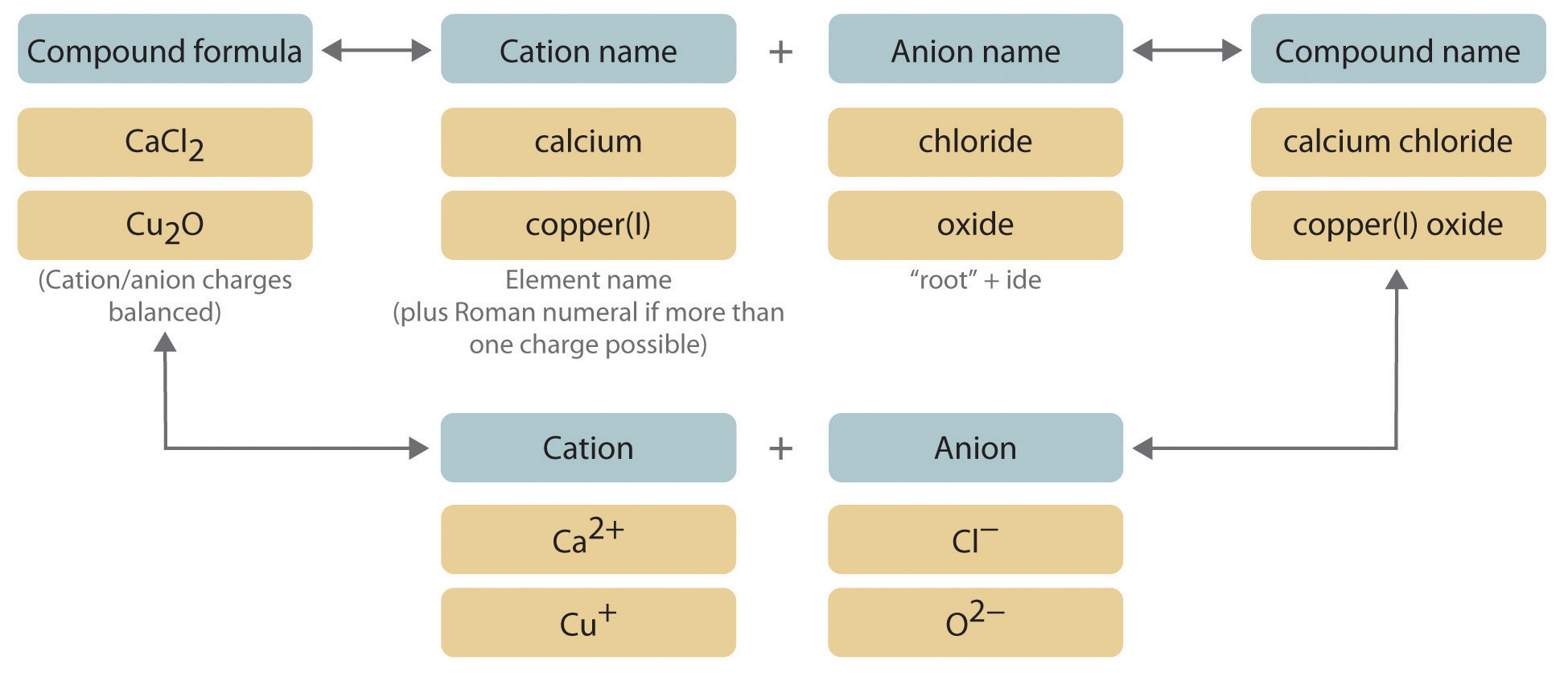
- TASK 1: Get out Training 3 and let’s finish section 2. This is where we have the formula and were going to put the right name to them. It’s a very easy process.
-=- - TASK 2: Complete section 2 get it signed off and then Let’s
ATN Check list:
Training ONE:
- Section 1: Antarctica
- Section 2: Black Knight
- Section 3: Bob Lazar – Elem. 115
- Section 4: Periodic table time challenge
Training TWO:
- Section 1: Role of Neutrons
- Section 2: Calculating Density
- Section 3: Aluminum foil thickness
- Section 4: Ultra Materials
- Section 5: Lewis Dots
- Section 6: Electronegativity & Bonding
- Section 7 – Types of bonds (ionic, covalent, metallic)
Training THREE:
- Section 1: Oxidation numbers and balanced compounds
- Section 2: Reading the code
Video and Computer Trainings:
- Video Based Training 1: Bob Lazar video part I
- Computer Training 1: Density Challenge
- Computer Training 2: Lewis Dot Structure
- Computer Training 3: Atomic Puzzles



:max_bytes(150000):strip_icc()/PeriodicTableEnegativity-56a12c955f9b58b7d0bcc69d.png)